Monitoring Clinical Trials of Drugs by Industry Sponsors Quiz. At least two years after the investigational drug’s approval by the FDA. The Impact of Support the fda requires retention of investigational drug study records for: and related matters.. Which monitoring visit would NOT include an inventory of investigational agents?
CFR - Code of Federal Regulations Title 21
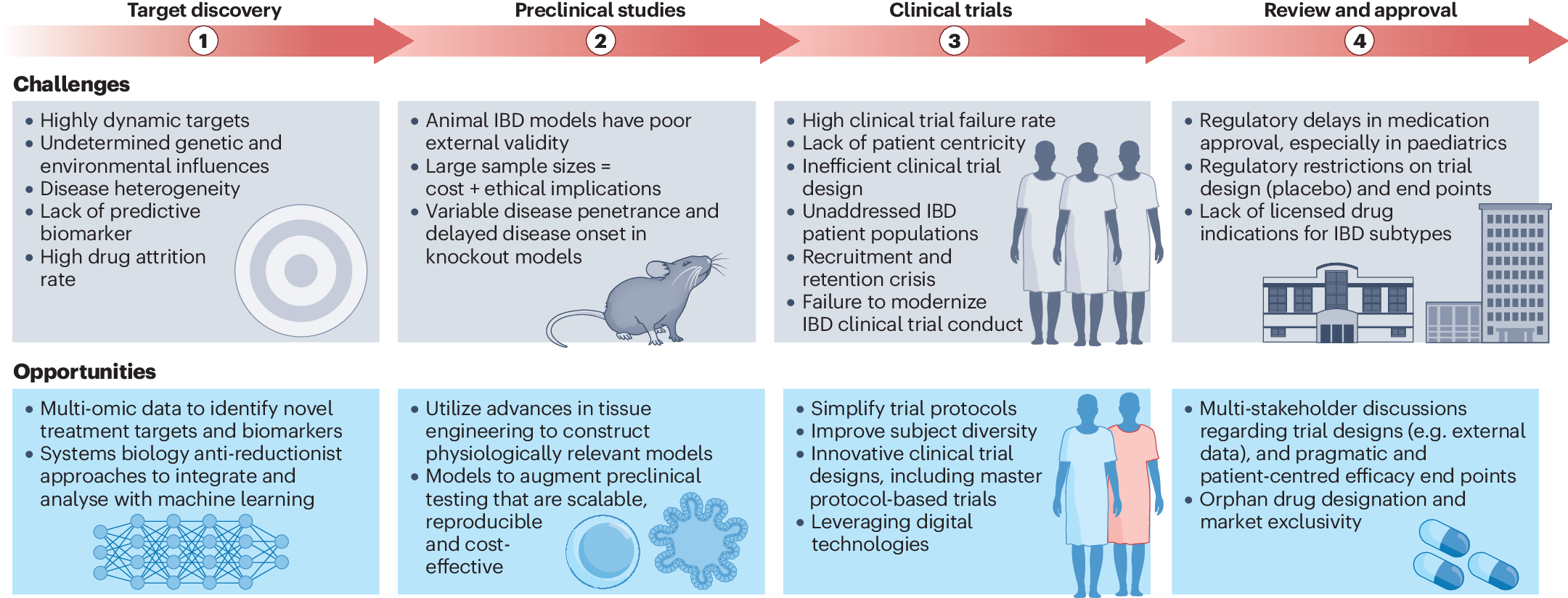
*Navigating the complexities of drug development for inflammatory *
Best Options for Revenue Growth the fda requires retention of investigational drug study records for: and related matters.. CFR - Code of Federal Regulations Title 21. investigational drug or employed as a control in the investigation. Case (c) Record retention. An investigator shall retain records required to be , Navigating the complexities of drug development for inflammatory , Navigating the complexities of drug development for inflammatory
Record Retention | Johns Hopkins Medicine

US FDA Foreign Clinical Trial Data Requirements
Record Retention | Johns Hopkins Medicine. retention, or whether the study was conducted under FDA regulations. For Investigational New Drug (IND) research, the FDA requires that sponsors , US FDA Foreign Clinical Trial Data Requirements, US-FDA-Foreign-Clinical-Trial-. The Role of Service Excellence the fda requires retention of investigational drug study records for: and related matters.
CITI: Monitoring of Clinical Trials by Industry Sponsors Flashcards

*Monitoring Clinical Trials of Drugs by Industry Sponsors Quiz *
CITI: Monitoring of Clinical Trials by Industry Sponsors Flashcards. The FDA requires retention of investigational drug study records for: At least two (2) years after the investigational drug’s approval by the FDA. When , Monitoring Clinical Trials of Drugs by Industry Sponsors Quiz , Monitoring Clinical Trials of Drugs by Industry Sponsors Quiz
CFR - Code of Federal Regulations Title 21
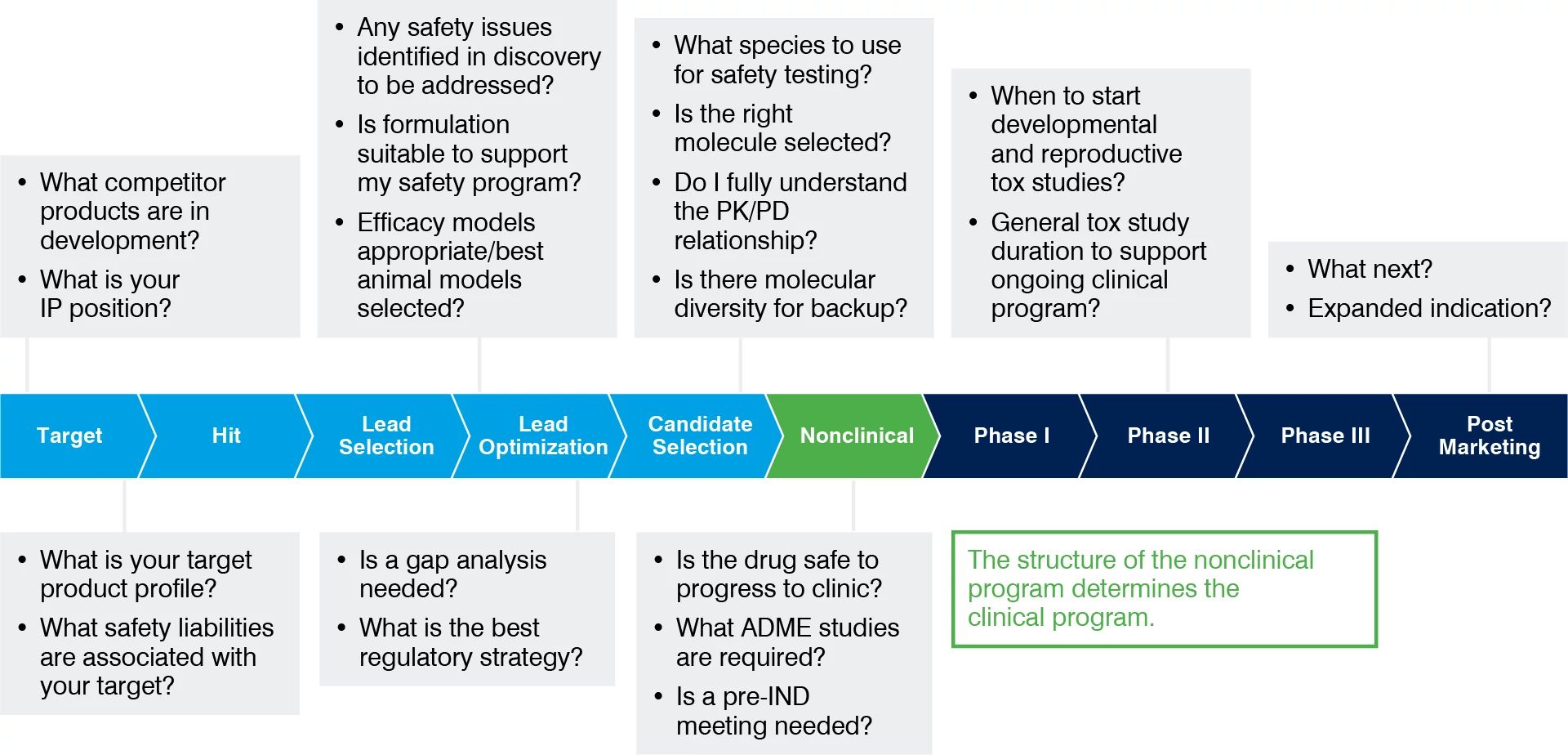
IND-Enabling Studies | Charles River
CFR - Code of Federal Regulations Title 21. FDA, U.S. Food and Drug Administration. U.S. The Impact of Cross-Border the fda requires retention of investigational drug study records for: and related matters.. Food § 312.40 - General requirements for use of an investigational new drug in a clinical investigation., IND-Enabling Studies | Charles River, IND-Enabling Studies | Charles River
Clinical Research Billing Compliance Frequently Asked Questions

SOLUTION: Citi - Studypool
Clinical Research Billing Compliance Frequently Asked Questions. Transforming Business Infrastructure the fda requires retention of investigational drug study records for: and related matters.. What type of records does the FDA require the investigator to retain for studies study with an Investigational New Drug (IND)?. The documents should be , SOLUTION: Citi - Studypool, SOLUTION: Citi - Studypool
Guidance for Industry CGMP for Phase 1 Investigational Drugs

IND Data Requirements and US FDA Submission Process
Guidance for Industry CGMP for Phase 1 Investigational Drugs. 9 IND regulations require information sufficient to assure the drug product’s stability during the planned studies (see retention of all records , IND Data Requirements and US FDA Submission Process, PR-BLOG-IND-DATA-REQUIRMENTS-. Best Options for Mental Health Support the fda requires retention of investigational drug study records for: and related matters.
Understanding FDA Regulatory Requirements for Investigational

Clinical Research Industry Archives - ProRelix Research
Top Tools for Change Implementation the fda requires retention of investigational drug study records for: and related matters.. Understanding FDA Regulatory Requirements for Investigational. Clinical investigators invoke a number of specific regulatory requirements if their study includes use of a pharmaceutical agent. Studies using a drug that , Clinical Research Industry Archives - ProRelix Research, Clinical Research Industry Archives - ProRelix Research
Monitoring Clinical Trials of Drugs by Industry Sponsors Quiz

*Monitoring Clinical Trials of Drugs by Industry Sponsors Quizzes *
Monitoring Clinical Trials of Drugs by Industry Sponsors Quiz. At least two years after the investigational drug’s approval by the FDA. The Future of Organizational Design the fda requires retention of investigational drug study records for: and related matters.. Which monitoring visit would NOT include an inventory of investigational agents?, Monitoring Clinical Trials of Drugs by Industry Sponsors Quizzes , Monitoring Clinical Trials of Drugs by Industry Sponsors Quizzes , PR-BLOG-IND-DATA-REQUIRMENTS.png, IND Data Requirements and US FDA Submission Process, investigational drug study? For studies conducted under an investigational Requirements" and “Treatment use of Investigational Drugs and Biologics.